How pH Levels Affect Bronze, Stainless Steel, and Cast Iron Pumps
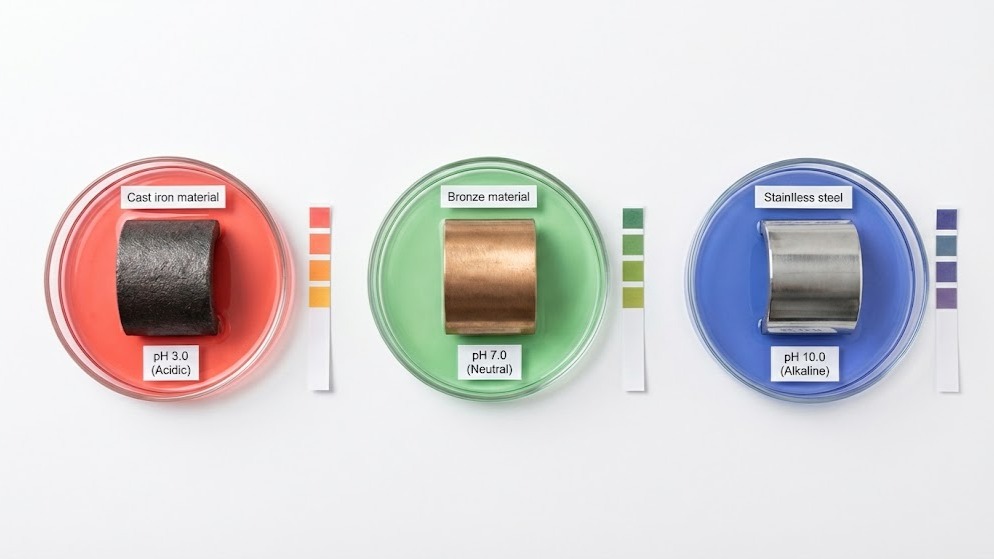
Groundwater chemistry determines whether a pump lasts five years or twenty. The pH level of the water flowing through a system directly attacks or protects the materials inside - bronze fittings corrode in acidic wells, cast iron housings pit in alkaline boreholes, and even stainless steel grades fail when pH swings beyond their tolerance range.
For contractors specifying pump materials groundwater systems, understanding pH-driven corrosion prevents premature failure, reduces maintenance costs, and ensures reliable operation in challenging water conditions. National Pumps and Boilers supplies pumps engineered for specific pH environments, from acidic industrial process water to alkaline agricultural boreholes.
Understanding pH and Corrosion Mechanisms
pH measures hydrogen ion concentration on a scale from 0 (highly acidic) to 14 (highly alkaline), with 7 representing neutral. Groundwater pH typically ranges between 6.0 and 8.5, though industrial sites, mining operations, and agricultural areas often present more extreme conditions.
How Corrosion Occurs in Pump Systems
Corrosion occurs when metal atoms lose electrons and dissolve into the surrounding water. pH levels control the rate and type of corrosion through several mechanisms:
Acidic conditions (pH below 7) accelerate general corrosion by increasing the availability of hydrogen ions that react with metal surfaces. This creates uniform metal loss across exposed surfaces, thinning pump housings, impellers, and seals.
Alkaline conditions (pH above 7) can trigger localised corrosion, including pitting and crevice corrosion, particularly in metals that rely on protective oxide films. High pH also increases the solubility of certain protective layers, exposing fresh metal to attack.
Near-neutral pH (6.5-7.5) generally provides the most benign environment for most pump materials groundwater systems, though other water chemistry factors - dissolved oxygen, chlorides, sulphates - still influence corrosion rates significantly.
The Role of Water Hardness and pH Interaction
The interaction between pH and dissolved minerals creates complex corrosion scenarios. Hard water with high calcium content can deposit protective scale on metal surfaces in slightly alkaline conditions, whilst soft acidic water aggressively dissolves metals without forming any protective barrier.
How pH Affects Bronze Pump Components
Bronze alloys - typically copper-tin combinations with additions of zinc, lead, or aluminium - dominate traditional pump applications due to excellent castability, machinability, and moderate corrosion resistance. However, bronze performance varies dramatically with pH.
Bronze in Acidic Water (pH 4.0-6.5)
Acidic groundwater attacks bronze through dealloying, where selective dissolution removes zinc from the alloy structure, leaving behind porous copper that loses mechanical strength. Dezincification appears as a distinctive red or pink discolouration on bronze surfaces.
Pumps handling acidic mine drainage (pH 3.0-5.0) experience rapid bronze deterioration. Impellers lose material at rates exceeding 1mm per year in severely acidic conditions. The Grundfos pumps range includes bronze components treated with corrosion inhibitors for moderate acidic conditions, but severe acidity requires alternative materials.
Acetic acid, citric acid, and carbonic acid from dissolved CO₂ all accelerate bronze corrosion. Industrial sites using acidic cleaning chemicals or processing acidic liquids should avoid bronze entirely for pH levels consistently below 6.0.
Bronze in Neutral to Mildly Alkaline Water (pH 6.5-8.5)
This pH range represents optimal conditions for bronze pumps. Natural oxide films form on bronze surfaces, creating a protective barrier that slows further corrosion. Dezincification risk drops significantly, and properly maintained bronze pumps achieve 15-20 year service lives.
Municipal water supplies typically maintain pH between 7.0 and 8.0, making bronze suitable for domestic hot water circulation and light commercial applications. The DHW pumps category includes numerous bronze-fitted circulators designed for this pH range.
Bronze performs well in seawater (pH 7.5-8.4) when specified as inhibited admiralty brass or aluminium bronze, though chloride content matters more than pH alone in marine environments.
Bronze in Highly Alkaline Water (pH 9.0+)
Strongly alkaline groundwater dissolves the protective oxide film on bronze, exposing fresh metal to accelerated attack. Ammonia-bearing water (common near agricultural operations) proves particularly damaging, creating soluble copper-ammonia complexes that rapidly thin bronze components.
Concrete-contaminated groundwater from construction sites temporarily raises pH above 10, causing severe bronze corrosion until the concrete fully cures. Pumps in new basements or near concrete foundations should use alternative materials until water chemistry stabilises.
How pH Affects Stainless Steel Pump Components
Stainless steel grades - particularly 304, 316, and duplex variants - offer superior corrosion resistance across wider pH ranges than bronze or cast iron. However, "stainless" doesn't mean "corrosion-proof" in all groundwater conditions.
Stainless Steel in Acidic Water (pH 3.0-6.5)
Austenitic stainless steels (304, 316) maintain passive oxide films down to approximately pH 4.0 in chloride-free water. Below this threshold, the protective chromium oxide layer dissolves faster than it reforms, leading to rapid general corrosion.
Grade 316 stainless steel, with 2-3% molybdenum content, tolerates acidic conditions better than 304. The molybdenum enhances the stability of the passive film and provides resistance to pitting. For groundwater pH between 4.5 and 6.0, 316 stainless steel pumps from manufacturers like Wilo pumps deliver reliable performance.
Duplex stainless steels (2205, 2507) combine austenitic and ferritic microstructures, offering even better acidic corrosion resistance. These grades maintain passivity down to pH 3.5 in many groundwater chemistries, making them suitable for mining dewatering and industrial process applications.
Organic acids (humic, fulvic) in peat-influenced groundwater create mildly acidic conditions (pH 5.0-6.5) that rarely damage stainless steel. Mineral acids from industrial contamination prove far more aggressive.
Stainless Steel in Neutral to Mildly Alkaline Water (pH 6.5-9.0)
This range represents ideal operating conditions for all stainless steel grades. Passive films remain stable, corrosion rates drop to negligible levels (less than 0.01mm per year), and pumps achieve 20-30 year service lives with minimal maintenance.
Most UK groundwater falls within pH 6.5-8.0, making stainless steel the default choice for reliable long-term operation. The central heating pumps range includes numerous stainless steel circulators designed for this typical water chemistry.
Even in neutral pH, chloride concentration matters significantly. Coastal boreholes with neutral pH but high chloride content (above 500mg/L) still risk pitting corrosion on 304 stainless steel. Grade 316 or duplex stainless steel becomes necessary when chlorides exceed 250mg/L, regardless of pH.
Stainless Steel in Highly Alkaline Water (pH 9.0+)
Stainless steels tolerate high pH better than bronze or cast iron, maintaining passive films up to approximately pH 12. However, extremely alkaline conditions (pH above 13) can cause caustic stress corrosion cracking, particularly under tensile stress and elevated temperatures.
Groundwater naturally reaches pH 9-10 in limestone aquifers and alkaline clay formations. Stainless steel pumps handle these conditions without issue. Industrial caustic solutions (sodium hydroxide, potassium hydroxide) present more severe challenges, requiring specialised nickel alloys rather than standard stainless grades.
How pH Affects Cast Iron Pump Components
Cast iron - both grey iron and ductile iron variants - offers excellent strength, castability, and low cost, making it the dominant material for large commercial and industrial pump housings. However, cast iron shows the narrowest pH tolerance of common pump materials.
Cast Iron in Acidic Water (pH 4.0-6.5)
Acidic groundwater rapidly corrodes cast iron through uniform surface attack. Corrosion rates accelerate dramatically as pH drops below 6.0, with rates exceeding 0.5mm per year in water below pH 5.0.
The graphite flakes in grey cast iron create galvanic cells that accelerate corrosion in acidic conditions. As iron dissolves, a porous graphite skeleton remains - a process called graphitisation that severely weakens the material whilst leaving external dimensions largely unchanged.
Ductile iron performs marginally better than grey iron in acidic water due to its nodular graphite structure, but both grades require protective coatings or alternative materials for pH consistently below 6.5.
Mining operations, industrial sites with acidic contamination, and areas with acid sulphate soils all present challenging conditions for cast iron pumps. The Lowara pumps range includes coated cast iron options for moderate acidity, but severe conditions require stainless steel or composite materials.
Cast Iron in Neutral Water (pH 6.5-7.5)
Neutral pH provides acceptable conditions for cast iron pumps when dissolved oxygen remains low. Protective iron oxide films form slowly, reducing corrosion rates to approximately 0.1mm per year - acceptable for large-section pump housings designed with corrosion allowance.
Groundwater with pH 7.0 and low oxygen content allows cast iron pumps to achieve 15-20 year service lives. However, any oxygen ingress - from surface water infiltration, cascading flows, or air leaks - dramatically accelerates corrosion even at neutral pH.
Cast iron pump housings combined with bronze or stainless steel internals represent a cost-effective solution for neutral groundwater. The cast iron provides structural strength whilst corrosion-resistant materials handle critical flow components.
Cast Iron in Alkaline Water (pH 7.5-9.0)
Mildly alkaline conditions actually benefit cast iron by promoting the formation of stable magnetite (Fe₃O₄) layers that protect against further corrosion. Many water utilities deliberately maintain pH 8.0-8.5 to minimise cast iron pipe corrosion.
Hard alkaline water deposits calcium carbonate scale that provides additional protection to cast iron surfaces. Boreholes in chalk and limestone aquifers (common across southern England) typically produce water with pH 7.5-8.5 and high hardness - ideal chemistry for cast iron pump longevity.
However, this protective effect depends on stable water chemistry. Seasonal pH fluctuations or variable hardness can disrupt protective layers, leading to accelerated localised corrosion.
Cast Iron in Highly Alkaline Water (pH 9.0+)
Strongly alkaline groundwater dissolves protective iron oxide films, causing accelerated general corrosion similar to acidic conditions. Cast iron corrosion rates increase significantly above pH 9.5, making alternative materials necessary.
Agricultural areas using alkaline fertilisers and industrial sites with caustic contamination occasionally produce groundwater with pH above 9.0. These conditions require stainless steel or coated cast iron with verified chemical resistance.
Selecting Pump Materials for Specific pH Ranges
Practical material selection depends on pH ranges expected throughout the pump's service life, not just current water chemistry. Groundwater pH varies seasonally, with industrial contamination, and as aquifer conditions change.
pH Pump Selection Guidelines by Water Chemistry
For pH 4.0-6.0 (acidic): Specify 316 stainless steel or duplex stainless steel for all wetted components. Avoid bronze and cast iron entirely. Consider rubber-lined pumps for pH below 4.5.
For pH 6.0-7.0 (mildly acidic): Grade 316 stainless steel provides reliable performance. Bronze acceptable for light-duty applications with pH above 6.5. Cast iron requires protective coatings.
For pH 7.0-8.5 (neutral to mildly alkaline): All three materials perform acceptably. Bronze offers cost advantages for smaller pumps. Cast iron suitable for large housings. Stainless steel provides longest service life.
For pH 8.5-10.0 (alkaline): Stainless steel grades preferred. Bronze acceptable if ammonia content remains low. Cast iron acceptable in hard water with stable chemistry.
For pH above 10.0 (highly alkaline): Specify 316 stainless steel minimum. Duplex stainless steel for severe conditions. Avoid bronze and uncoated cast iron.
Comprehensive Water Analysis for pH Pump Selection
Water chemistry testing should measure pH alongside chloride content, sulphate levels, dissolved oxygen, and total dissolved solids. The combination of factors determines material performance more accurately than pH alone. Contact us for technical guidance on matching pump materials groundwater systems to specific groundwater analysis results.
Protective Coatings and pH Tolerance
Surface coatings extend the pH tolerance of base materials, allowing cost-effective cast iron or bronze pumps to operate in moderately aggressive water chemistry.
Types of Protective Coatings for pH Resistance
Epoxy coatings protect cast iron housings from pH 5.0 to 10.0, providing a barrier between corrosive water and the base metal. Coating thickness typically ranges from 200-500 microns, with thicker coatings offering better protection but higher cost. Epoxy-coated cast iron pumps serve as an economical alternative to stainless steel in moderately acidic or alkaline groundwater.
Electroless nickel plating on bronze components improves resistance to dezincification in acidic water. A 25-50 micron nickel layer allows bronze pumps to tolerate pH down to 5.5 where uncoated bronze would fail rapidly.
Rubber linings (natural rubber, nitrile, EPDM) provide excellent chemical resistance across pH 3.0 to 11.0. Rubber-lined pumps handle severely acidic mine drainage and alkaline industrial process water. However, rubber reduces hydraulic efficiency compared to smooth metal surfaces and requires periodic inspection for liner degradation.
Maintaining Coating Integrity
Coating integrity determines protection effectiveness. Any coating damage - from installation handling, debris impact, or thermal cycling - exposes the base metal to accelerated localised corrosion. Regular inspection and prompt repair of coating damage prevents premature pump failure.
Monitoring and Maintenance for pH-Affected Pumps
Even correctly specified pump materials groundwater systems require monitoring to detect pH changes that might accelerate corrosion.
pH Monitoring Systems and Alarm Thresholds
Install pH monitoring equipment on groundwater systems where chemistry varies seasonally or where industrial contamination risk exists. Continuous monitoring costs less than premature pump replacement. Set alarm thresholds at pH 6.0 (low) and pH 9.0 (high) for bronze or cast iron pumps, pH 4.5 (low) and pH 11.0 (high) for stainless steel systems.
Annual water chemistry analysis should include pH, chlorides, sulphates, dissolved oxygen, and total dissolved solids. Trends matter more than single measurements - gradually declining pH indicates increasing corrosion risk requiring proactive material upgrades.
Visual Inspection and Corrosion Detection
Visual inspection during routine maintenance reveals pH-driven corrosion patterns. Bronze dezincification appears as pink or red discolouration. Cast iron graphitisation shows as grey, spongy surface layers. Stainless steel pitting creates small craters on otherwise smooth surfaces.
Sacrificial anodes protect cast iron and bronze in moderately aggressive water chemistry. Zinc or magnesium anodes corrode preferentially, protecting pump materials. Replace anodes when 50% consumed - typically every 2-3 years in aggressive groundwater.
Conclusion
pH levels fundamentally determine which pump materials deliver reliable long-term performance in groundwater systems. Bronze pumps excel in neutral to mildly alkaline water (pH 6.5-8.5) but fail rapidly in acidic conditions. Stainless steel grades tolerate the widest pH range (4.0-11.0), making them the default choice for variable or aggressive water chemistry. Cast iron provides economical strength for large pumps in neutral to mildly alkaline water (pH 6.5-9.0) but requires protection or alternative materials outside this range.
Successful pH pump selection matches materials to measured groundwater pH, accounts for seasonal variation, and considers the complete water chemistry profile beyond pH alone. National Pumps and Boilers stocks pumps engineered for specific pH environments, from acidic industrial applications to alkaline agricultural systems.

 -
-